ePRO

From bottlenecks to breakthroughs: How AI is transforming translation timelines
According to ClinicalTrials.gov, there are 3,046 multi-country trials being conducted this year. While many trials remain localized within a single country, there has been a definitive movement towards conducting trials in multiple countries, especially for larger, later-stage trials. This is driven by the positives that multi-country trials offer, like faster patient recruitment, lower costs in some regions, and the need for diverse patient populations.. However, behind the scenes, a critical bottleneck has been slowing many trials down. This bottleneck is the translation process that’s required to make trials work across multiple languages, locales, and regulatory bodies/organizations.


The Top 5 myths about eCOA in 2025
With MarketsandMarkets projecting the global electronic Clinical Outcome Assessments (eCOA) solutions market to grow at compound annual growth rate (CAGR) of 16.1% each year until 2030, it’s clear the eCOA has made its impact within the clinical research landscape.
Yet despite growing adoption, electronic Clinical Outcome Assessments (eCOA) are still surrounded by misconceptions that hinder their full potential. From concerns about patient usability to assumptions about cost and implementation timelines, these myths can create hesitation among sponsors and research teams alike.
Thus, we’re here to cut through the noise and set the record straight with a summary of a recent webinar featuring speakers from Transcelerate and Medable.


Accelerating oncology research: Digital strategies for modern clinical trials
Learn how digital tools ease oncology research for all stakeholders while accelerating the pace of research with this whitepaper.


Tips for better eCOA experiences from our Patient Caregiver Network
Electronic Clinical Outcome Assessments (eCOA) have revolutionized data collection in clinical trials, offering convenience and real-time insights that traditional methods cannot match.
However, the effectiveness of these digital tools hinges on the quality of the daily experience they offer participants. Read below to see direct feedback from patients and caregivers across multiple conditions in our Patient Champion Network (PCN), as we identify ten critical factors that can improve the eCOA experience.


The science behind Medable’s HEOR
“No one knows their level of pain and how they feel or function better than the patient. Capturing the patient’s response to an electronic Patient Reported Outcome (ePRO) is always the best option.”
Since its inception, Medable has prioritized scientific integrity and rigor around various aspects of clinical trials. As part of its science team, Cindy Howry, VP of eCOA Science, plays a crucial role in ensuring that Medable’s clinical trial platform accurately captures electronic Clinical Outcome Assessments (eCOAs). Hired in 2023, Howry’s chief purpose at Medable is to ensure that the health economics and outcomes research (HEOR) science behind our technology is based on best practices and regulatory guidance.

Explore Medable Studio
A decade ago, our founders’ experiences in clinical research and life sciences drove them to create Medable, a simple cloud-based platform that could improve theconduct of trials. Over the last ten years, we’ve conducted nearly 400 trials on our platform, continually refining it to provide a better experience for sites, patients, and caregivers.Today, we’re excited to announce that our customers are entering a new era of control, ease of use, and simplicity.


Clinical Leader Solutions Expo: eCOA/ePRO
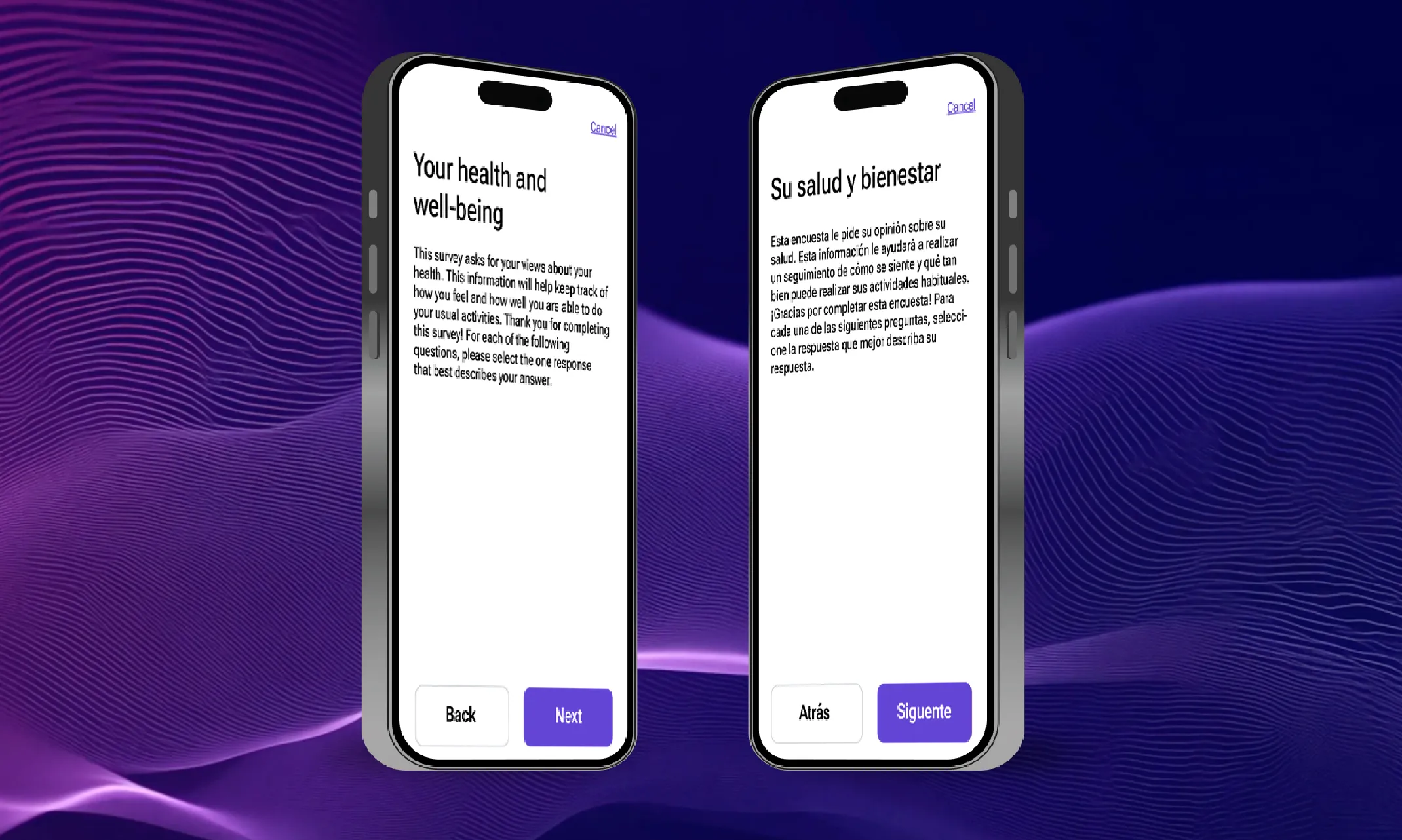
The next level of clinical trial data capture is here. Gone are the days of paper, illegible handwriting, and data quality nightmares. eCOA, or electronic Clinical Outcome Assessments, and ePRO, or electronic Patient-Reported Outcomes, are revolutionizing the way clinical trials generate data. Both leverage digital tools to collect information electronically, offering a treasure trove of benefits. eCOA acts as the umbrella term, encompassing data from various sources like patients (ePRO), clinicians, observers, and even performance-based assessments. This holistic approach paints a more complete picture of a patient's experience. ePRO, as a subset, shines the spotlight directly on the patient's voice, capturing their symptoms, quality of life, and other critical self-reported measures.
Hear from industry leaders at this unique virtual event showcasing the eCOA/ePRO solutions of partners who are ready to help you with your clinical trial data capture needs. Early registration is open via the form to your right.


Defining “evidence generation” within modern clinical trials
Medable has always been, at its core, a platform that enables clinical trial sponsors to collect data and generate evidence to answer scientific questions.
But from the start, Medable has done things differently. Today, the flexibility, purpose, and build of Medable’s evidence-collection platform is what sets us apart from others in our space.

Taking eCOA technology deployment off the critical path
Within the context of clinical trials, the "critical path" refers to the sequence of activities, tasks, or events that, if delayed or extended, would directly impact the overall timeline for the trial.
These essential steps and dependencies must be completed in a specific order to ensure the trial stays on schedule. Any delay in the critical path can potentially lead to a delay in the entire trial, affecting its completion date and potentially increasing costs. Identifying and managing the critical path is crucial for efficient trial management and timely delivery of treatments to patients.
For a simpler example, take a look at how Monday.com describes the critical path within the context of building a house and their visual aid in Exhibit 2. “For example, if you’re building a house, the critical path might include digging the foundations, building the walls, and installing the roof. If any of these critical activities fall behind schedule the whole project gets delayed.”


Amplifying Evidence with Unified Clinical Trial Data Collection
Join us for a discussion about how combining a unified clinical trial data collection platform with intelligent automation can revolutionize drug development and why the current status quo is no longer sufficient. With novel capabilities, the industry can now explore new possibilities, such as incorporating additional sensors to identify surrogate or alternative endpoints, rethinking adaptive trial designs, and quickly responding to emerging data.


You asked we answered: How do eCOAs improve data compliance?
We at Medable are often asked in webinars, requests for proposals (RFPs), tradeshows, etc. just how exactly eCOAs and ePROs foster better patient compliance with submitting data.
Since you asked, our answer is below, courtesy of Jessica Dolfi, VP of Solution Consulting.


ePROs: Transforming oncology trial research
Over the last decade the number of oncology trials has skyrocketed, almost doubling the number of all other therapeutic areas combined, according to the WIRB-Copernicus Group¹. Known for their complex design, oncology trials often present various participant, site, and sponsor hurdles.
Sponsors and CROs looking to tackle these challenges andreduce the burden on participants and sites should explore the potential of digital solutions, particularly electronic informed consent (eConsent) and electronic patient reported outcomes (ePRO). Both tools offer expanded views of the participant journey while offering feedback that enables sponsors and CROs to enhance and refine their trials for all stakeholders.


Back to basics: What are wearables and how are they powering patient diversity and efficiency in eCOA
Mobile health technology, like medical-grade wearabledevices, can transform patient monitoring by enabling the collection of newtypes of data and better accuracy of patient responses. This tech can increaseaccessibility of trials for patients and even increase diversity and reducepatient burden by removing geographical and travel barriers.
As a result of these myriad benefits, we’ve seen theincreasing inclusion and acceptance of wearable devices throughout clinicaltrials. Here, we’ll dive into the specifics of these helpful tools and theirimpact on the future of medicine.



