
Groundbreaking approach, Faster progress.
Accelerate therapeutic discovery and maximize ROI with our streamlined platform, fully integrated across your entire portfolio.
Standardize across your entire portfolio
Unlock better ROI with our platform-wide solutions. With features like standardized workflows, single sign-on and IRT Integration, you’ll see increased efficiency and scalability in your trials.
The Medable platform enables 24/7 accessibility and multi-study use that spans across your entire organization.
Scalable solutions
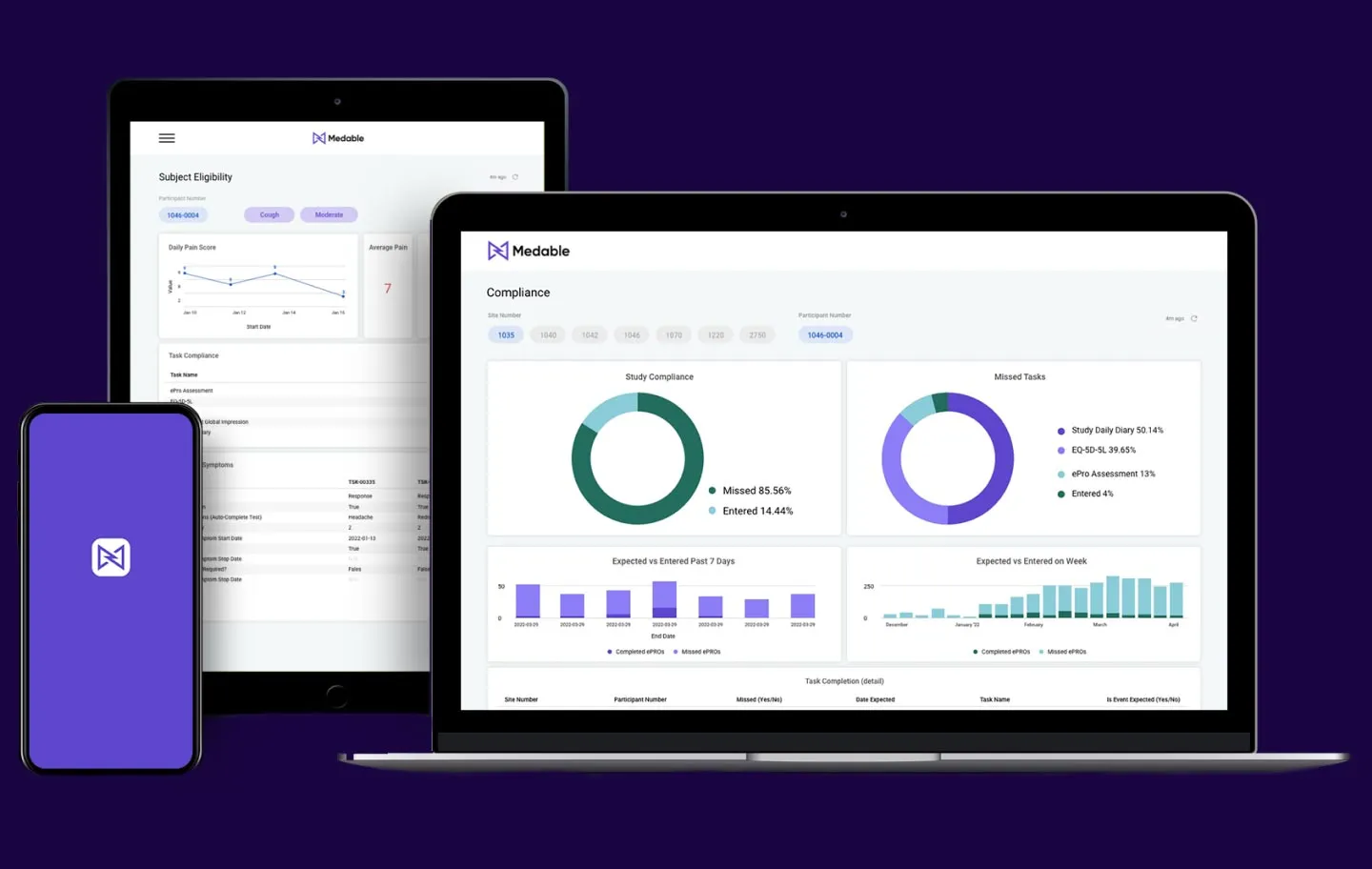
The power of scale
Build your tools once, them use them again. Medable enables reusability standards so that building and implementing future trials is faster and easier.
Make every moment count
With one complete view of data across your portfolio, you’ll make faster, more informed decisions saving time and making a difference where it matters.


Extensive DCT Network
Do more with your study by leveraging our extensive network of partners ranging from retail pharmacy and site networks, data providers for RWE and LTFU, and direct-to-patient offering of connected sensors and home health providers.
Trusted by



The latest from Medable


Technology Overload: Addressing Site Challenges of Digital Trials
Katie madden, Digital & Process Optimization at GSK, joins Medable’s Andrew MacKinnon and Annie Hesslewood to discuss the importance of implementing a change management strategy coupled with a unified digital trial platform that streamlines eConsent, eCOA, sensors, and integration workflows into a single end-to-end experience.


Use case: Implementing digital solutions to improve oncology trial experience & efficiency
A top 10 pharmaceutical company looked to Medable to streamline participant burden and enhance site and team experience in oncology trials, while establishing a scalable template for future trials in oncology and other areas.


What it really takes to adopt eConsent across large pharma
Learn about the benefits of adopting consent management technology and best practices around developing change management and training programs to help sponsors, CROs and sites get the most out of eConsent.



