AI and ML


Build vs buy: A guide on adopting AI agents for life sciences
“Big corporations can’t rely on their internal speed to match the transformation that is happening in the world. As soon as I know a competitor has decided to build something itself, I know it has lost.”
These candid sentences from Sanofi CEO, showcase one of the most common questions that’s at the forefront of every pharmaceutical company’s mind; whether to build or buy your way into the agentic and generative AI revolutions.
In life sciences, many teams start with the same instinct. They see a capable large language model, stand up a proof of concept, and feel close to a breakthrough. For most of us, AI prototypes can look magical. A chatbot summarizes visit reports, drafts emails, or answers protocol questions in minutes. The experience is so strong that teams assume production is a short step away.
Unfortunately, the gap is much bigger than it looks.
According to a recent MIT study, 95% of AI pilots will fail, as they note that “Only 5% of custom GenAI tools survive the pilot-to-production cliff, while generic chatbots hit 83% adoption for trivial tasks but stall the moment workflows demand context and customization.”
Like MIT’s example shows, moving from prototype to production in clinical research means building something validated, compliant, scalable, and integrated into real workflows. That takes far more than clever prompts. It requires domain grounding, continuous monitoring, retraining loops, robust tool orchestration, and evidence that the system is safe and auditable under regulations like GxP, HIPAA, and 21 CFR Part 11.
Many organizations only discover the hidden costs after they have committed. Internal teams often invest for two years, spend millions in sunk cost, and still never reach a dependable clinical grade system. The illusion comes from how easy it is to get an early demo working, and how hard it is to make that demo survive contact with trial reality.


Sponsors talk AI: Sanofi's take on the evolving role of AI in clinical trials
Artificial intelligence continues to move from experimentation to execution across the life sciences industry. On a recent episode of the AI in Business podcast, Matthew Peruhakal, Global Head of Data Architecture, Utilization, and AI Engineering at Sanofi, offered a deep look at how the pharmaceutical giant is integrating AI to transform clinical trials. From intelligent data workflows to proactive risk detection and regulatory alignment, Peruhakal described an organization reshaping its research and development operations around a new digital core.
His message was clear: AI cannot remain a side project. To make a meaningful impact, it must be embedded as a strategic capability that connects people, systems, and data across the enterprise.


Sponsors talk AI: Takeda’s take on the evolving role of AI in clinical trials
Artificial intelligence continues to influence nearly every industry, and life sciences are no exception. In a recent conversation on the AI and Business podcast, Damien Nero, Head of Data Science in US Medical at Takeda Pharmaceuticals, shared his perspective on how AI is changing the clinical trial landscape. With over 15 years of experience applying machine learning and real-world data to drug development, Nero outlined both the progress already being made and the challenges that still stand in the way of broader transformation. His insights highlight how pharmaceutical leaders can think strategically about deploying AI to balance innovation with operational efficiency.


Rewriting the Future of Clinical Trials: AI, Agility, and the Portfolio-First Mandate
In a rapidly changing research landscape, leaders at Takeda, Novartis, Sanofi, Daiichi Sankyo, and Medable share firsthand insights into how artificial intelligence is reshaping clinical trial operations.
This exclusive whitepaper explores how forward-thinking organizations are reimagining trial design, execution, and scaling to meet the demands of speed, precision, and patient-centricity across their entire portfolio.

From bottlenecks to breakthroughs: How AI is transforming translation timelines
According to ClinicalTrials.gov, there are 3,046 multi-country trials being conducted this year. While many trials remain localized within a single country, there has been a definitive movement towards conducting trials in multiple countries, especially for larger, later-stage trials. This is driven by the positives that multi-country trials offer, like faster patient recruitment, lower costs in some regions, and the need for diverse patient populations.. However, behind the scenes, a critical bottleneck has been slowing many trials down. This bottleneck is the translation process that’s required to make trials work across multiple languages, locales, and regulatory bodies/organizations.


Back to basics: Agentic AI and how it’s impacting clinical trial research
Since the release of OpenAI’s ChatGPT in 2022, the buzz around artificial intelligence has been impossible to ignore. From advertisements during the SuperBowl to webinars and working groups, the impact of artificial intelligence has been felt in almost every sector of our world.
But, what if we told you the most transformative shift is still on the horizon?
When ChatGPT first released it changed the way the world, including clinical research, worked. Now NVIDIA, one of the most premier companies leading the way in the development of AI, has stated that they expect the development of Agentic AI, a new type of artificial intelligence to “change the way we work in ways that parallel how different work became with the arrival of the internet.”
This means agentic AI may have a much bigger impact than even generative AI did years back.
So, if you’re curious about agentic AI, read on as we delve into its nature, differentiate it from generative AI, and reveal its transformative role in clinical research."


Recapping DIA 2025
The 2025 Drug Information Association (DIA) Global Annual Meeting, held in Washington D.C., is beginning to wind down. As always, the conference has left a clear vision for the future of clinical trials. one defined by groundbreaking innovation, unprecedented global collaboration, and a profound commitment to patient well-being. This year's conference underscored key themes that are shaping the landscape of medical product development, with Artificial Intelligence (AI) and Real-World Data (RWD) taking center stage.


Vibe coding is real: The rise of the developer as agent manager
By Tim Smith, Co-Founder & CTO,Medable
In today’s rapidly evolving tech landscape, vibe coding is opening up exciting opportunities by blending human expertise with smart agents to give every engineer incredible leverage and productivity. This new approach invites us to embrace innovative tools that drive greater scale and impact. But it’s not enough to just pick up new practices—success demands a significant mental shift and a change in approach. Developers must move beyond their traditional roles as individual contributors and evolve into “agent managers.” This means blending deep technical expertise with the strategic oversight of a manager, actively coaching intelligent agents so that each interaction builds on the last and contributes to a cohesive, scalable solution.


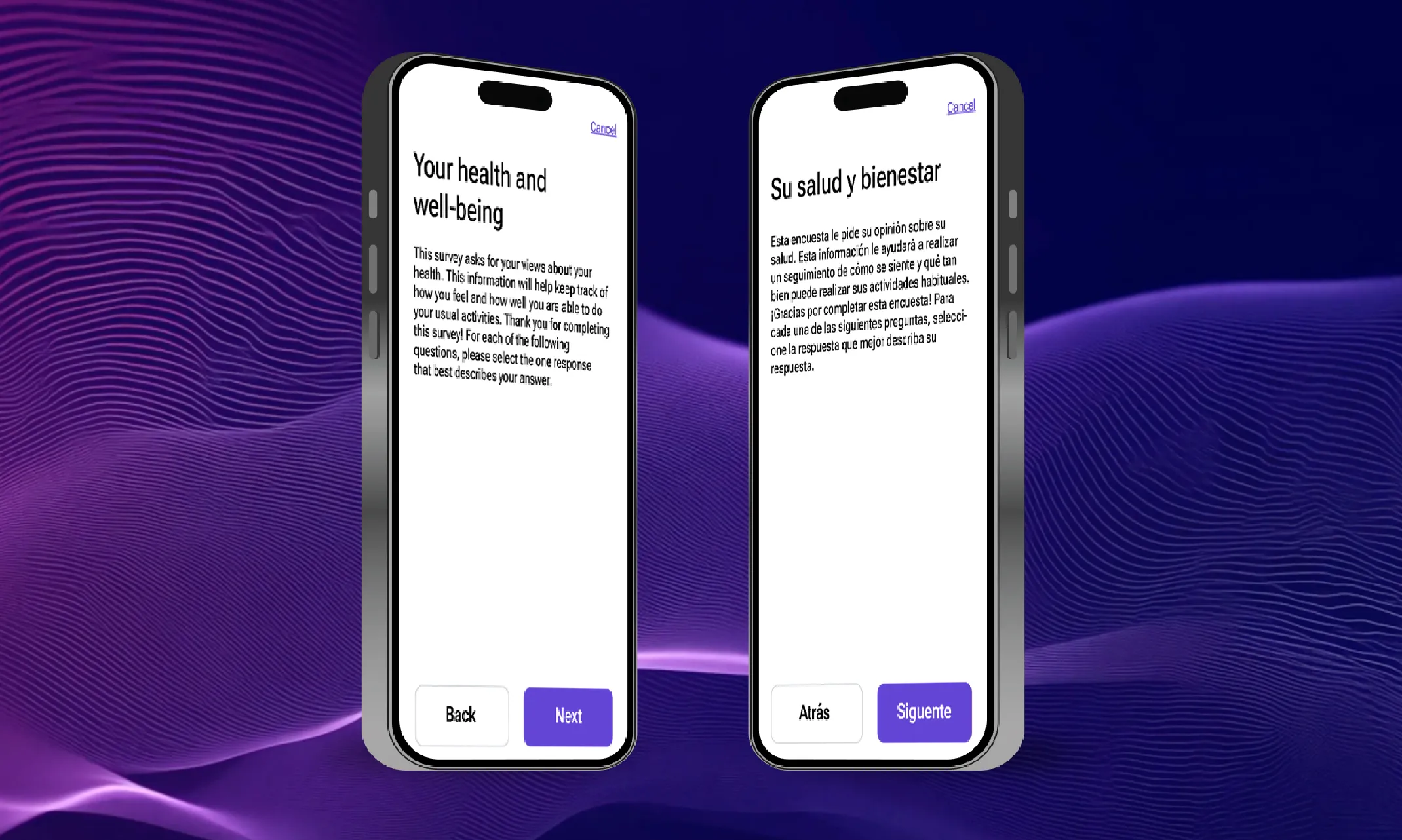
Instant eCOA generation, refinement, and scaling with Medable AI
Watch the latest demo that shows you how to create eCOAs in seconds with Medable Studio and AI.

Transforming clinical trials: The impact of eCOA on trial design
Discover how electronic Clinical Outcome Assessments (eCOA) are revolutionizing clinical trial design. This post explores how digital tools enhance patient engagement, improve data accuracy, and drive the shift toward decentralized clinical trials. Dive in to see why integrating eCOA is essential for modern, efficient clinical research.


SCOPE 2025: Trends, insights, and news
This year’s Scope Summit once again brought thousands in the clinical research space together, offering a glimpse into the evolving operational and technological landscapes shaping the future of trials.

J.P.M. Week 2025
The J.P. Morgan Healthcare Conference returned to San Francisco this year, bringing significant deals and strategic shifts that signal where healthcare is headed. While this year’s weather outside was dry and sunny, a substantial change from the prior two years' rainy conferences, the conditions inside differed, with several companies making waves.


Clinical Leader Solutions Expo: eCOA/ePRO
The next level of clinical trial data capture is here. Gone are the days of paper, illegible handwriting, and data quality nightmares. eCOA, or electronic Clinical Outcome Assessments, and ePRO, or electronic Patient-Reported Outcomes, are revolutionizing the way clinical trials generate data. Both leverage digital tools to collect information electronically, offering a treasure trove of benefits. eCOA acts as the umbrella term, encompassing data from various sources like patients (ePRO), clinicians, observers, and even performance-based assessments. This holistic approach paints a more complete picture of a patient's experience. ePRO, as a subset, shines the spotlight directly on the patient's voice, capturing their symptoms, quality of life, and other critical self-reported measures.
Hear from industry leaders at this unique virtual event showcasing the eCOA/ePRO solutions of partners who are ready to help you with your clinical trial data capture needs. Early registration is open via the form to your right.



