BioTech


What does the new European Medicine Regulatory Network recommendation paper on decentralized elements in clinical trials tell us?
Learn what impacts the new European Medicine Regulatory Network recommendations paper means to your clinical trial operations.



New EU Regulatory Recommendations for Decentralized Trials Keep Focus on Patients and Sites
As clinical trials rapidly modernize, government regulators work to evolve even as the same core principles apply. Still, decentralized clinical trials are – simply – clinical trials, as all research today is decentralized in some way, yet we lack global harmonization of regulations.
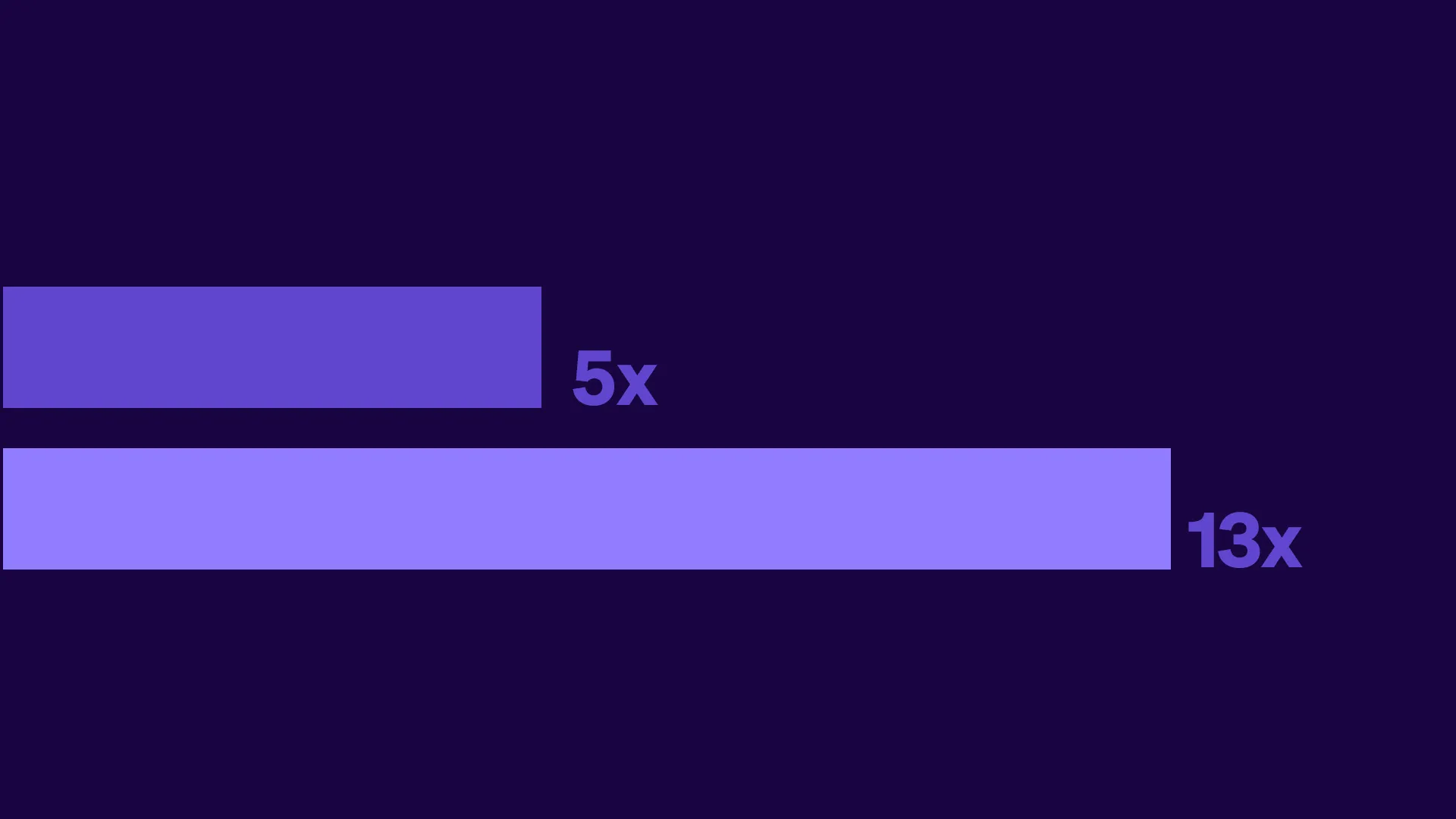

Tufts CSDD Impact report: Decentralized and hybrid trials deliver greater ROI than traditional trials
The Tufts Center for the Study of Drug Development completed an analysis of its financial modeling study of decentralized clinical trials using real data from Medable-enabled studies. These findings net financial benefits ranging from 5x for Phase II and 13x for Phase III trials, equating to roughly $10 million ROI and $39 million ROI, respectively.




