ePRO

From bottlenecks to breakthroughs: How AI is transforming translation timelines
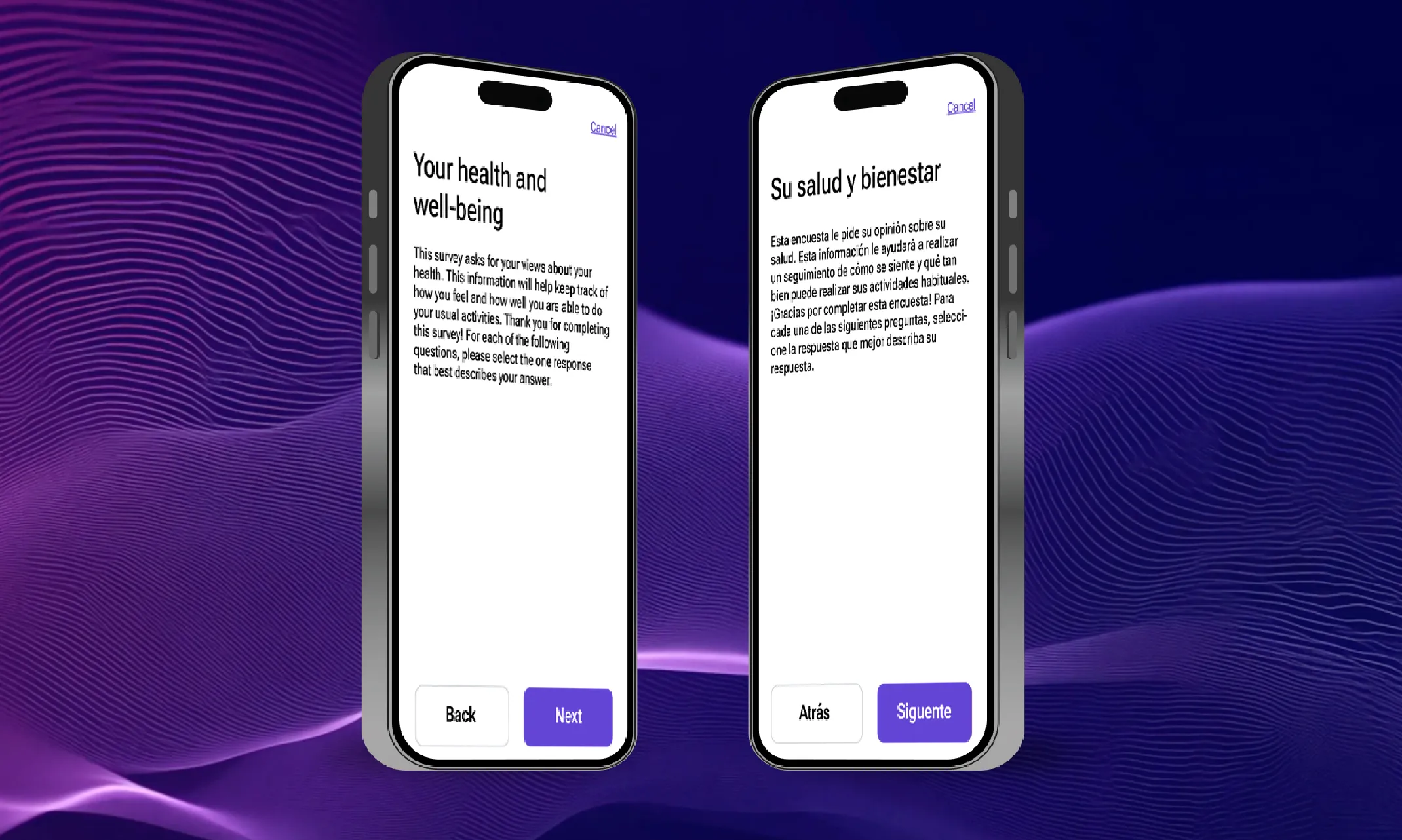
According to ClinicalTrials.gov, there are 3,046 multi-country trials being conducted this year. While many trials remain localized within a single country, there has been a definitive movement towards conducting trials in multiple countries, especially for larger, later-stage trials. This is driven by the positives that multi-country trials offer, like faster patient recruitment, lower costs in some regions, and the need for diverse patient populations.. However, behind the scenes, a critical bottleneck has been slowing many trials down. This bottleneck is the translation process that’s required to make trials work across multiple languages, locales, and regulatory bodies/organizations.


Back to basics: What are electronic clinical outcome assessments (COAs/eCOAs)?
COAs (called eCOAs when captured electronically) are essential to understanding whether a drug is reducing symptoms, improving patients’ quality of life, and improving patients’ ability to perform activities they care about. COAs enable a well-rounded understanding of how a drug is working, its side effects, its impact on patients’ lives, and more. Perhaps most notably, PROs/ePROs allow for the patient’s voice to be heard. Capturing the patient’s voice is particularly important because the clinician may not always see or express the patient’s experience in the same way.

Back to Basics: What is a decentralized clinical trial?
In a decentralized clinical trial, part or all of the protocol occurs away from the primary study site. Instead of patients traveling, often repeatedly, to a central site for enrollment, consent, data collection or symptom monitoring, they can participate in telehealth visits from their homes, often using familiar technologies, like smartphones, tablets and wearables to transmit pertinent information. Even medications and devices can increasingly be delivered directly to a patient’s home, and a home visit from a health care professional can be arranged if necessary.


Leverage novel options in oncology clinical trial design to reduce burden for patients and sites
Flo Mowlem, Senior Director, eCOA Science & Solutions shares insights on how electronic solutions, with a focus on patient-reported outcome (ePROs) can reduce burden on participants and sites to maximize the chance of success in oncology clinical trials.


ePROs: Transforming oncology research
In this webinar, VP of Digital Trial Solutions Musaddiq Khan, VP of Product Strategy Colin Weller, and Cancer Survivor Lindsey Matt discuss how Medable is supporting a patient-first approach in complex Oncology clinical trials


Designing patient-friendly ePRO instruments
This blog explores why patient-centric ePRO instrument design is critical to driving study success, provides tips on how to make instruments more patient-centric, and offers resources for additional ePRO design guidance.





