Mid to Large Pharma


Case Study: Medable platform delivers >90% eCOA adherence and scalability
A top-10 American pharmaceutical company came to Medable looking to conduct an extensive weight management master protocol clinical trial with multiple sub-studies across 70+ research sites. The client was concerned about the participant experience, as the trial required participants to enroll through the master protocol before enrolling into a sub-study. Additionally, they wanted to reuse and scale the solution for future trials without compromising the site and patient experience.
Learn how Medable was able to accomplish this and more.


Use case: Implementing digital solutions to improve oncology trial experience & efficiency
A top 10 pharmaceutical company looked to Medable to streamline participant burden and enhance site and team experience in oncology trials, while establishing a scalable template for future trials in oncology and other areas.


What it really takes to adopt eConsent across large pharma
Learn about the benefits of adopting consent management technology and best practices around developing change management and training programs to help sponsors, CROs and sites get the most out of eConsent.


What does the new European Medicine Regulatory Network recommendation paper on decentralized elements in clinical trials tell us?
Learn what impacts the new European Medicine Regulatory Network recommendations paper means to your clinical trial operations.


New EU Regulatory Recommendations for Decentralized Trials Keep Focus on Patients and Sites
As clinical trials rapidly modernize, government regulators work to evolve even as the same core principles apply. Still, decentralized clinical trials are – simply – clinical trials, as all research today is decentralized in some way, yet we lack global harmonization of regulations.


The digital future is now
Watch the on-demand panel discussion between Medable, Syneos, Illingworth and Veeva, moderated by Gilead, as they discuss how the next generation of clinical development is now.
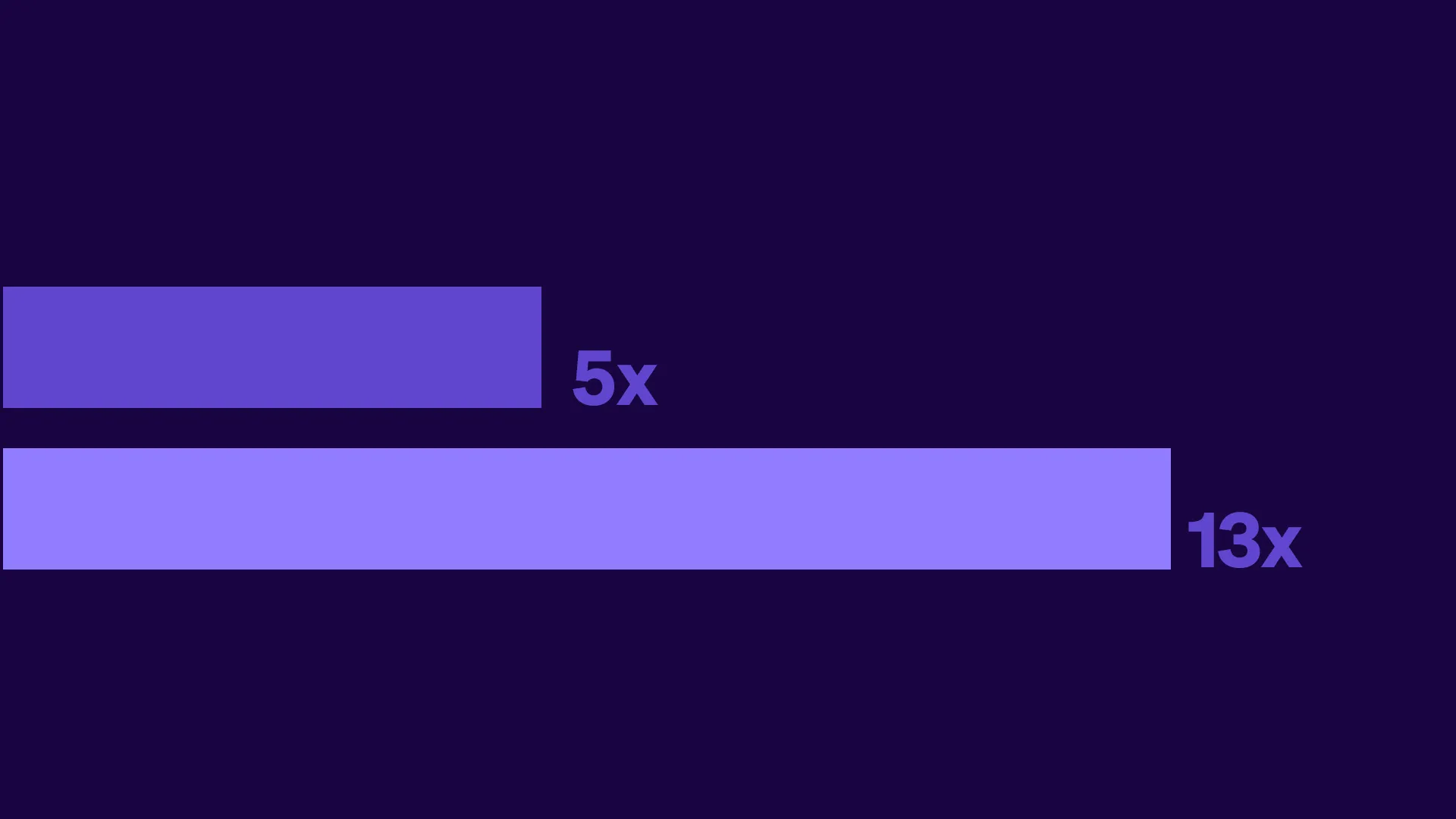

Tufts CSDD Impact report: Decentralized and hybrid trials deliver greater ROI than traditional trials
The Tufts Center for the Study of Drug Development completed an analysis of its financial modeling study of decentralized clinical trials using real data from Medable-enabled studies. These findings net financial benefits ranging from 5x for Phase II and 13x for Phase III trials, equating to roughly $10 million ROI and $39 million ROI, respectively.


Building trust between clinical trial stakeholders through a DCT framework
Industry experts from Pfizer, Advarra, and Medable showcase how to design a framework of decentralized offerings that provide greater ease of use, effectiveness, and flexibility for sites for every type of research setting; decentralized/hybrid, virtual, or in-person. In addition, they discuss how DCT solutions and site partners are creating an impact to improve diversity and access while optimizing clinical research for patients.



