
Faster PI review with human judgment in control
The PI Summary & Review Agent acts as an intelligent assistant that continuously monitors participant data and prepares the PI for review and sign-off.

“Regulators expect satisfactory oversight of remotely captured data, which traditionally forced PIs to verify content across multiple reports and systems. Our agent centralizes that effort for eCOA data, summarizing participants' most recent eCOA data, making remote oversight seamless.”
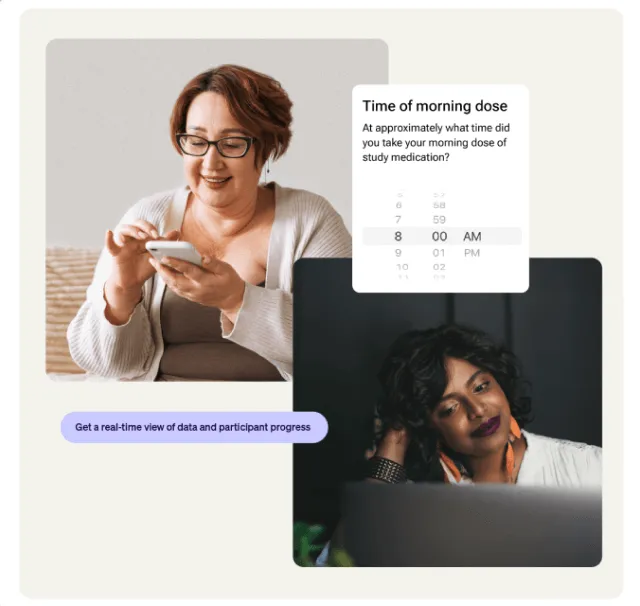
Real-time eCOA data review readiness
Step in, see the full picture, and review with complete control. Continuously monitor and organize participant data so PI reviews are always up to date.
Surfaces underlying data for complete transparency
Summaries are backed by full participant data. Review responses, draft changes, and make updates before signing, with a clear. view of exactly what was reviewed and when.

eCOA data review without disruption
Review and sign-off happen without blocking site or participant activity. Trials keep moving while oversight stays on track.
The latest from Knowledge Centers


Build vs buy: A guide on adopting AI agents for life sciences
“Big corporations can’t rely on their internal speed to match the transformation that is happening in the world. As soon as I know a competitor has decided to build something itself, I know it has lost.”
These candid sentences from Sanofi CEO, showcase one of the most common questions that’s at the forefront of every pharmaceutical company’s mind; whether to build or buy your way into the agentic and generative AI revolutions.
In life sciences, many teams start with the same instinct. They see a capable large language model, stand up a proof of concept, and feel close to a breakthrough. For most of us, AI prototypes can look magical. A chatbot summarizes visit reports, drafts emails, or answers protocol questions in minutes. The experience is so strong that teams assume production is a short step away.
Unfortunately, the gap is much bigger than it looks.
According to a recent MIT study, 95% of AI pilots will fail, as they note that “Only 5% of custom GenAI tools survive the pilot-to-production cliff, while generic chatbots hit 83% adoption for trivial tasks but stall the moment workflows demand context and customization.”
Like MIT’s example shows, moving from prototype to production in clinical research means building something validated, compliant, scalable, and integrated into real workflows. That takes far more than clever prompts. It requires domain grounding, continuous monitoring, retraining loops, robust tool orchestration, and evidence that the system is safe and auditable under regulations like GxP, HIPAA, and 21 CFR Part 11.
Many organizations only discover the hidden costs after they have committed. Internal teams often invest for two years, spend millions in sunk cost, and still never reach a dependable clinical grade system. The illusion comes from how easy it is to get an early demo working, and how hard it is to make that demo survive contact with trial reality.


What happened at ESMO AI & Digital 2025
The 2025 ESMO AI & Digital Oncology Congress, held in Berlin from November 12 to 14, highlighted the accelerating role of artificial intelligence across the oncology care continuum. Although imaging and pathology remain the most established fields for AI adoption, this year’s programming revealed a decisive shift toward workflow-integrated AI that enhances clinical operations, supports trial efficiency, and addresses the realities of patient monitoring.
Across three days of sessions, side-room conversations, and industry demonstrations, one theme was clear. AI is evolving from experimental add-on technology into a practical clinical teammate, but scaling its impact will require robust validation, seamless integration, and a sustained focus on clinician trust.






