Your co-pilot for clinical monitoring
Medable’s CRA Agent is an AI-driven solution that automates and optimizes clinical trial monitoring by proactively identifying and prioritizing site risks, generating comprehensive pre-visit summaries, and providing actionable recommendations to enhance trial oversight and compliance.
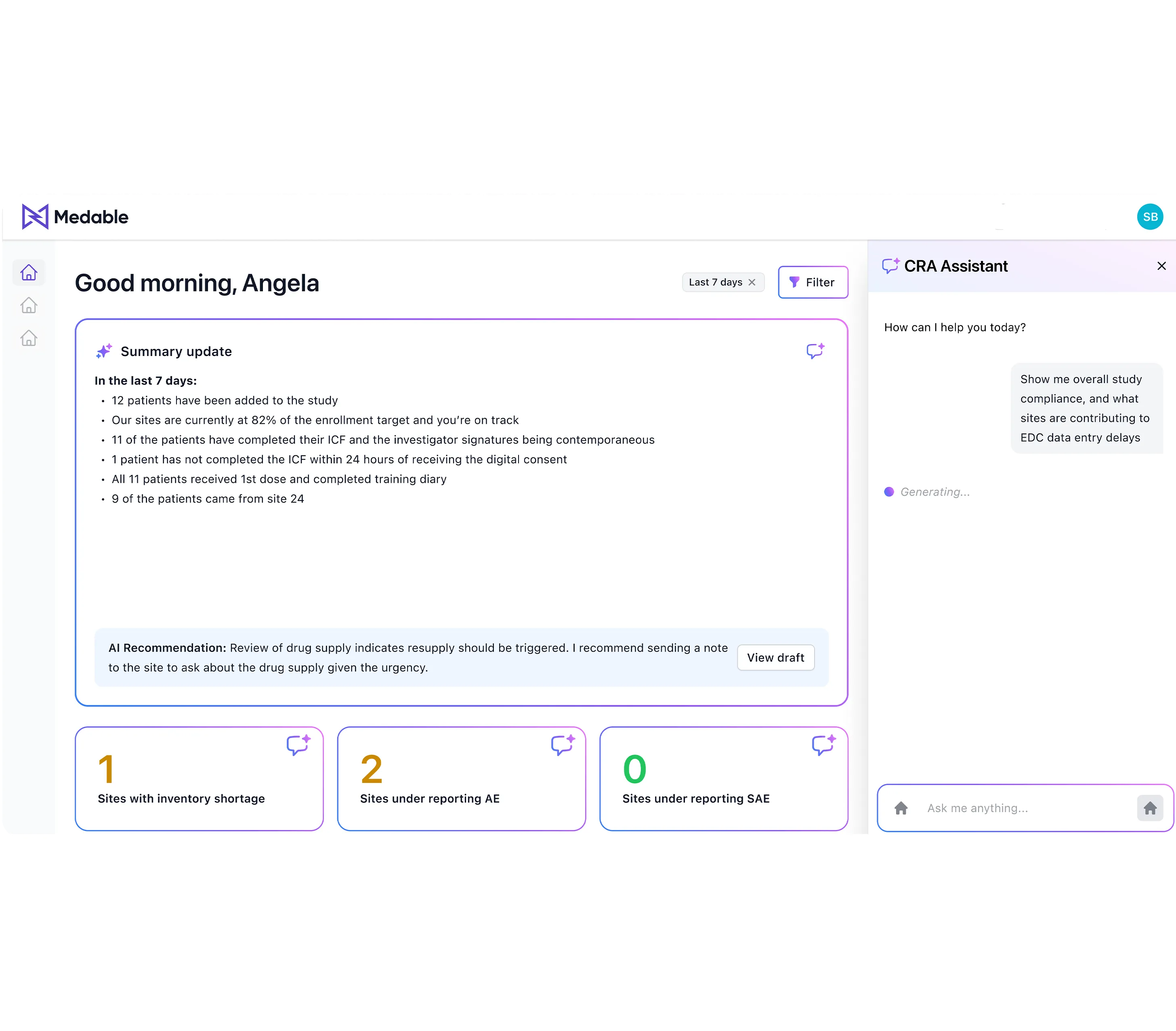

We estimate that CRA agents can take on up to 90% of the tactical and administrative work the CRA handles on a daily basis — from sending site-specific emails and reminders to tracking responses and updating systems. That means we can focus our time on the strategic decisions that move trials forward, while standardizing and streamlining the tasks that used to consume most of our day.

Connected monitoring across systems
Perceive signals across the entire clinical data ecosystem by unifying CTMS, RTSM, EDC, labs, consent, and safety into one view. Turn manual checks into automated insights, instantly highlighting which sites are on track and where intervention is needed, saving valuable time, system-hopping, and manual data compilation.
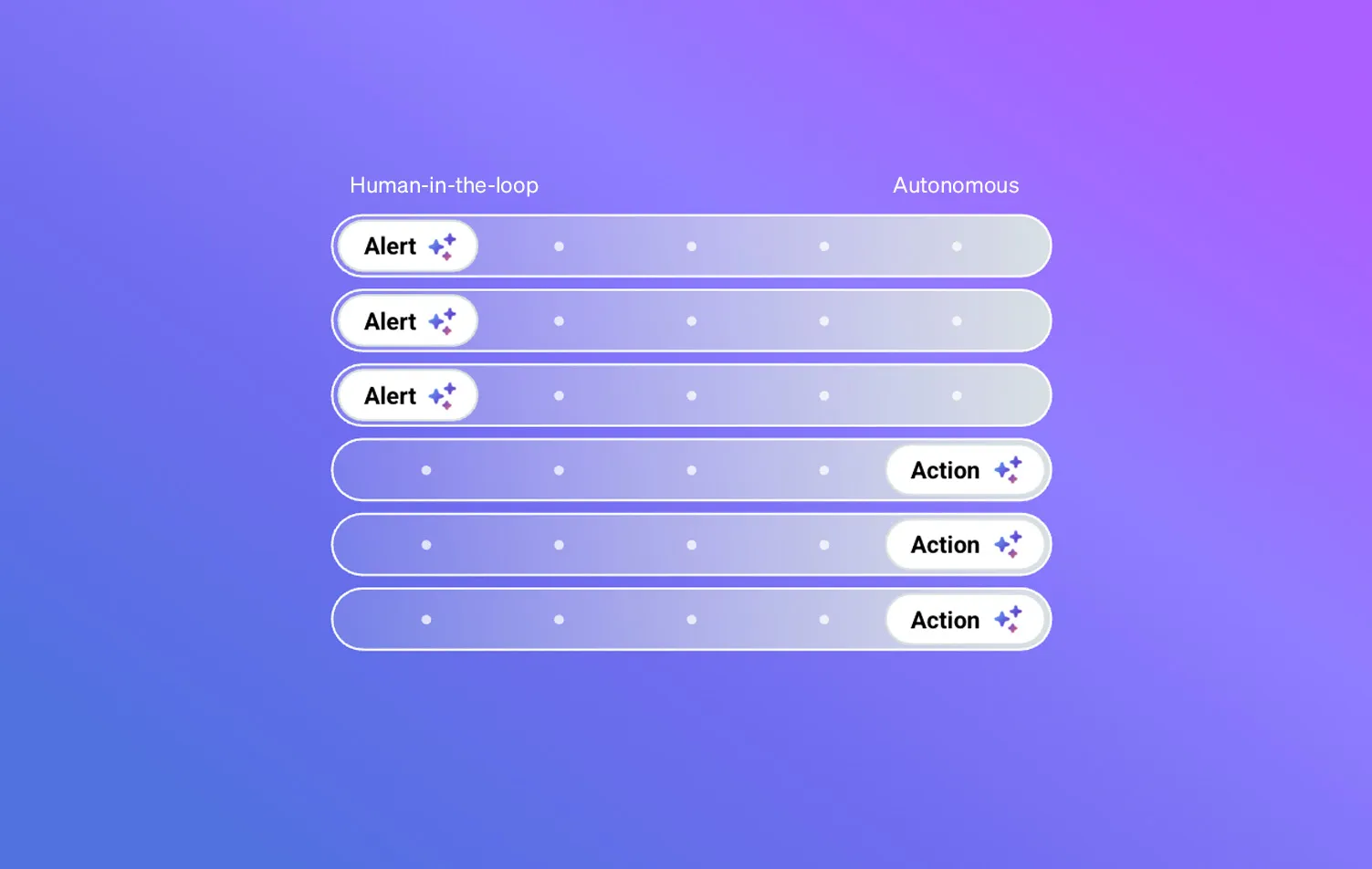
Human-in-the-loop oversight
Easily identify risks and recommendations for best-next actions tied to your protocol, consent, and regulatory requirements. Stay assured with a transparent reasoning trail thatshows why each step is advised, enabling CRAs to collaborate confidently with sites, improve decision-making, enhance their compliance, all while freeing CRAs to focus on engagement and site-specific challenges.
Automated administrative tasks
Take advantage of automated routine tasks like drafting queries, sending emails, and updating CTMS/eTMF — always with human CRA oversight. Close the loop between planning and execution with real-time tracking that reduces administrative burden, ensures data consistency, and accelerates trial progress.
The latest from Knowledge Centers

Cell and Gene Day 2026- Virtual Summit
Join experts to discuss how new FDA frameworks and increased pharma investment are shaping what’s next for cell and gene therapy.

Smart Sourcing: EDC, eCOA, & Data Management Showcase
Join us for a 2-hour virtual expo designed to help clinical trial professionals discover and compare the best Electronic Data Capture (EDC), electronic Clinical Outcome Assessment (eCOA), & Data Management technologies on the market, without the time, cost, or travel of traditional conferences.


Compounding interest: Why “good enough” data is good enough for agentic AI
Let’s ask a trick question.
Do you think your organization’s data is ready for AI, or AI Agents?
Most sponsors and CROs instinctively answer “not yet.” What this really means is that they don’t believe their data isn’t fully centralized, dictionaries aren’t perfectly aligned, and too many systems still operate in parallel. The result is that AI gets parked on the roadmap, waiting for a future state where everything is clean, standardized, and coordinated.
Here’s the twist; waiting for that moment is very thing holding organizations back.
When it comes to implementing agentic AI, the bigger risk right now isn’t imperfect data. Instead, it’s waiting for perfection before acting.

Eliminate clinical trial white space with the right AI strategy
It has become clear that our industry has reachedthe limits of human-only clinical development. As clinical trials have become increasingly complex, the endeavors that people alone can perform are no longer sufficient to generate the momentum needed to address the growing burden of human disease. This has led to longer drug development timelines and significant delays for patients. One large are of lost time is “white space,” definied simply as unproductive time caused by manual, sequential processes and fragmented data systems. Thankfully, a solution lies in agentic AI and its abilities to perform series of tasks.



