Documents organized. Audits cleared.
Medable’s eTMF agents take on the high-volume work of organizing and filing trial documents, operating continuously across studies so teams can move from manual execution to oversight.
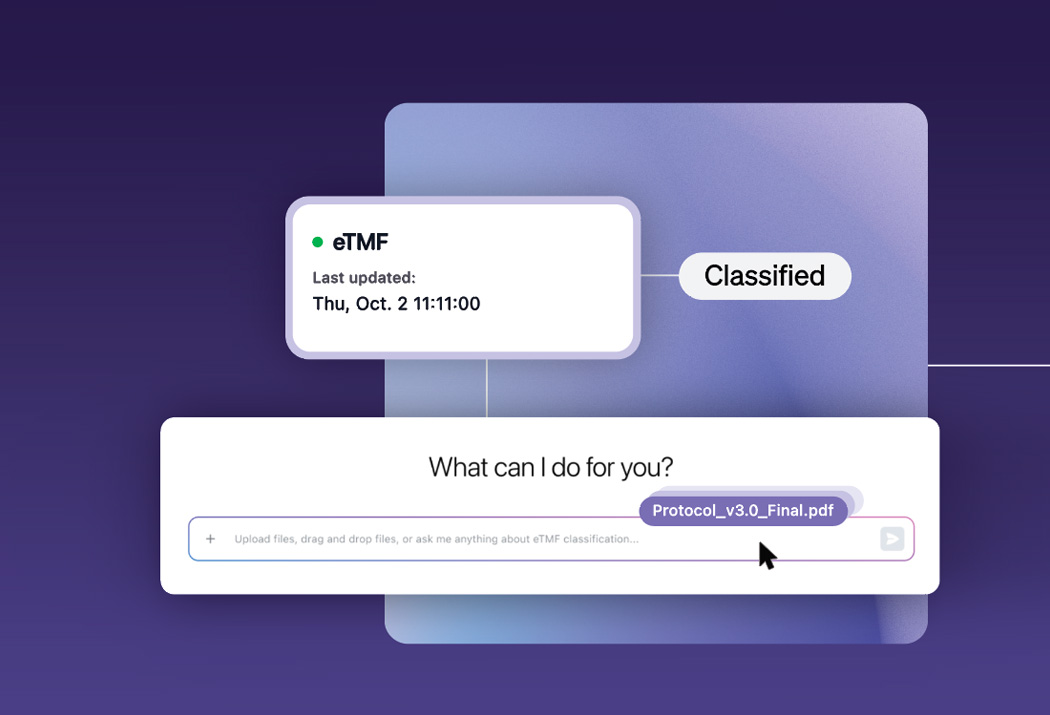
Automation without the blind spots
Automate document intake from inbox to eTMF, with intelligent classification, metadata extraction, and compliant filing—backed by confidence scoring and multilingual support for global scale.
Complete oversight
Agents handle the volume; your team reviews, approves, and stays in control where it matters.
Audit-ready by design
Every action is tracked with clear traceability, so documentation is inspection-ready without extra effort.
Fits with your TMF
Agentify your current TMF by automating how documents are organized, reviewed, and submitted.

Every file has to be checked, renamed, tagged, and uploaded by hand. It’s incredibly time-consuming, and even with good people, things get missed.
- 60% time saved per TMF document for classification
- Continuous document intake and organization
- Fewer backlogs and last-minute scrambles
- Designed to comply with all global regulations, including FDA 21 CFR Part 11, ICH E6 (R2), and GDPR
Frequently asked questions
Agents operate in a controlled environment with rigorous validation checkpoints, business logic guardrails, and real-time monitoring. Every agent is subject to verification workflows and data quality checks before being surfaced to users, ensuring accuracy, compliance, and auditability. Medable recommends human remain involved whenever the task carries higher risk.
All actions, prompts, responses, and connector events are logged within the platform. Once documents are filed in the target TMF system, that system’s native audit trail records the final action as well.
Each classification includes a confidence score. Users decide when to accept or adjust results.
The latest from Knowledge Centers


Medable enables over 90% eCOA adherence in vaccine trial
A leading biotech company came to Medable looking to conduct a vaccine trial. They were concerned that their trial’s participant population of persons aged 50+ years may be hesitant to use the sponsors’ chosen eCOAtrial technology, would not be engaged in the study, and therefore may not provide consistent trial data.
Learn how we drove success for them, including 90% eCOA adherence.


Bridging the gap: Ensuring sites are successful with Medable
For years, clinical research sites have been vocal about the technological challenges they face conducting trials. Obstacles such as multiple disparate systems per trial, an abundance of passwords, lack of interoperability, and other issues all showcase how technology can be as significant a hindrance as it is a boon. As a recent Forbes article suggests, if clinical research technology doesn’t empower sites, it risks slowing down the entire trial.


How we standardized deploying vaccine studies in five weeks
By Musaddiq Khan, VP, DCT Solutions
At Medable, we’re always looking to understand the changing environment of clinical research, and how we can help customers overcome the various challenges, risks, and scenarios that these changes bring.
For the past year, I, various clinicians, and research experts at Medable have been working closely to improve how we conduct research for various therapeutic areas. Much like the results shown in Tufts CSDD’s latest whitepaper, we believe that a well-thought, decentralized approach can help bring new therapies in historically challenging therapeutic areas to markets faster.
The result of this work is a brand-new decentralized approach we’re bringing to the market.




