ROI


The digital future is now
Watch the on-demand panel discussion between Medable, Syneos, Illingworth and Veeva, moderated by Gilead, as they discuss how the next generation of clinical development is now.







Deploying a patient-first framework to accelerate recruitment & reduce overall trial timelines
Learn how to utilize digital data-flow designs in clinical trials to improve patient diversity and inclusion, increase operational efficiencies, enhance patient enrollment, engagement, and retention, and realize financial value.
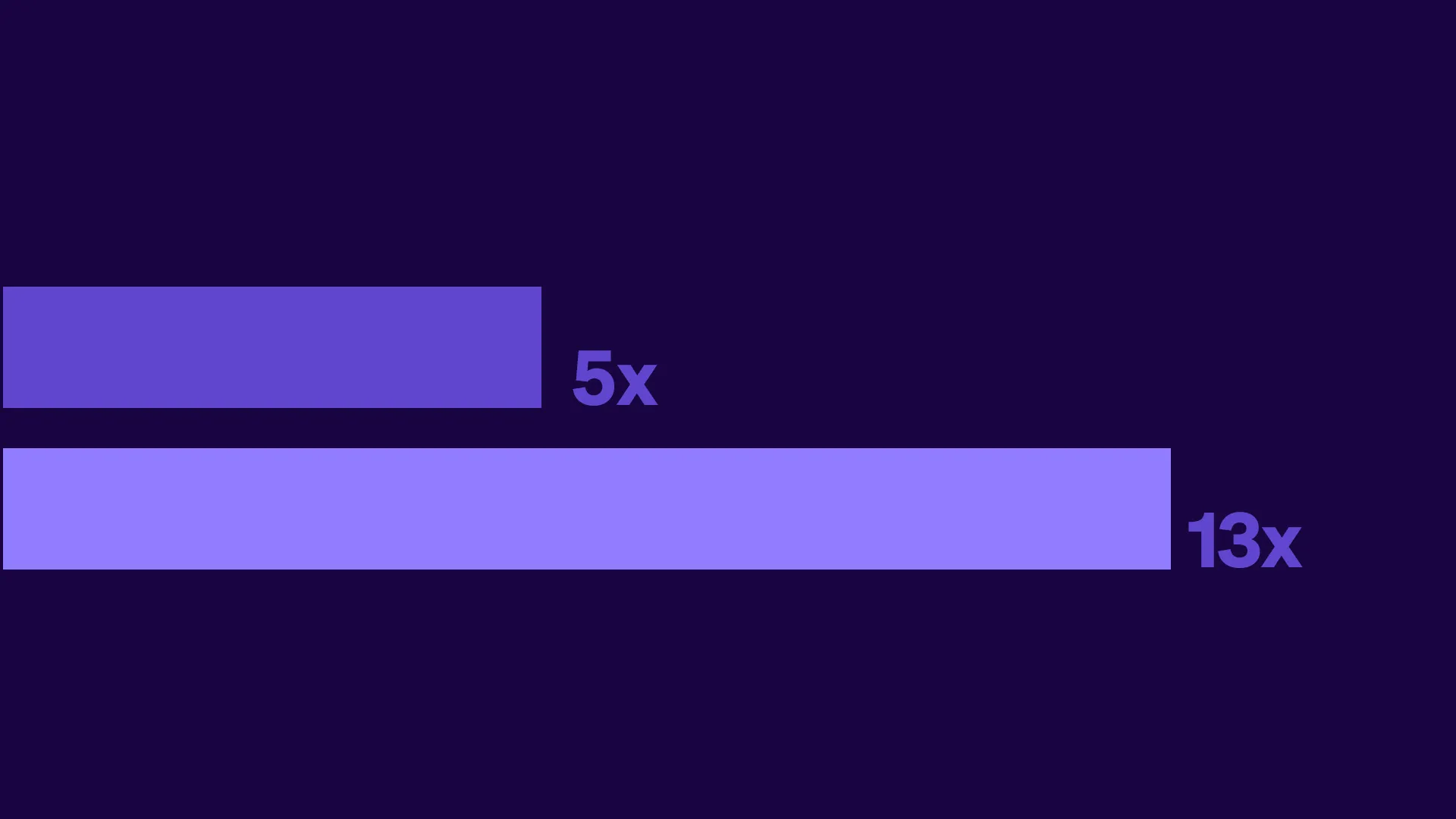

Tufts CSDD Impact report: Decentralized and hybrid trials deliver greater ROI than traditional trials
The Tufts Center for the Study of Drug Development completed an analysis of its financial modeling study of decentralized clinical trials using real data from Medable-enabled studies. These findings net financial benefits ranging from 5x for Phase II and 13x for Phase III trials, equating to roughly $10 million ROI and $39 million ROI, respectively.

Assessing the financial value of decentralized clinical trials
Deployment of remote and virtual clinical trial methods and technologies, referred to collectively as decentralized clinical trials (DCTs), represents a profound shift in clinical trial practice. To our knowledge, a comprehensive assessment of the financial net benefits of DCTs has not been conducted


DCT takes center stage at DIA 2022 with new Tufts CSDD Study
DIA 2022 provided four days worth of content spanning over 13 talk tracks and hundreds of speakers with three major takeaways.



