White Papers, Case Studies, Reports


Medable oncology solutions
Discover how Medable’s AI-powered oncology platform simplifies complex cancer trials by integrating eCOA, ePRO, and eConsent solutions—reducing trial time, improving patient retention, and enhancing data quality for faster, more efficient research.


Technology overload: Addressing site challenges in clinical research
In the early 2000s, a new era of clinical research began – the digital era. As large sponsors and CROs like IQVIA andGlaxo Smith Kline began implementing electronic data capture (EDC) systems into their trials, clinical trial sites experienced the first major digital disruption in how they conducted clinical research. Fast forward totoday, and this cycle of disruption not only continues, it’s shortened.
As a recent article by BusinessInsider states, “it took 30 years forelectricity to be adopted by 10% ofUS consumers. It took 25 years for the telephone to achieve the same penetration. Tablets achieved thisreach in just five years – and they’re not even our primary devices." This shortened cycle of mass adoption has brought smartphones,smartwatches, tablets, and moreto trials, as well as new software and portal applications like eCOA and eConsent. However, it’s been reported that too much technology is commonplace and it quickly overwhelms.
In this white paper, we’ll examine best practices in ensuring sites are adequately prepared and supported for new trial technologies,derived from the experiences of key individuals with direct experience from pharma, technology vendors and importantly from sites themselves.


Quality by Design: Better data using participant insights
Quality is not an afterthought within clinical trials. Instead, it is a required and integral part of the entire process beginning at the start of protocol design. While clinical trials will always encounter challenges in driving quality experiences and data, there exists one approach that’s backed by regulatory agencies and proven to lead to better outcomes.
This is a “quality by design” strategy that’s informed and driven by all stakeholders, including those most affected by your trial’s protocols - your participants, and sites.


Medable’s Patient Caregiver Network improves post-seizure trial data
A pharmaceutical contract research organization (CRO) expressed concerns about the timing and availability of seizure diaries for patients enrolled in their rare disease study.
They wanted to understand when patients should be prompted to complete the diaries and how they could improve the process to better accommodate caregivers' and patients' needs to produce better trial data.


Record-breaking enrollment achieved in weight-loss study
A top 10 pharmaceutical company approached Medable seeking support for their Phase III diabetes study. Their primary goal was to reduce the participant enrollment phase timeline to get the study underway as quickly as possible.

Medable platform speeds diabetes study startup by 50%
A top 10 pharmaceutical company approached Medable seeking support for their Phase III diabetes study in the highly competitive weight loss market. Their primary goal was toreduce the client’s average study build timelines from 16-20 weeks, down to just 8 weeks,a reduction of more than 50%.
See how Medable was able to meet the customer's goal with this case study.


ePROs: Transforming oncology trial research
Over the last decade the number of oncology trials has skyrocketed, almost doubling the number of all other therapeutic areas combined, according to the WIRB-Copernicus Group¹. Known for their complex design, oncology trials often present various participant, site, and sponsor hurdles.
Sponsors and CROs looking to tackle these challenges andreduce the burden on participants and sites should explore the potential of digital solutions, particularly electronic informed consent (eConsent) and electronic patient reported outcomes (ePRO). Both tools offer expanded views of the participant journey while offering feedback that enables sponsors and CROs to enhance and refine their trials for all stakeholders.

The Digital Future is Now eBook
According to Grandview Research,the hybrid and decentralized clinicaltrial (DCT) market will be worth more than 12 billion dollars by 2030. Sparked by the COVID-19 pandemic of 2020, the rapid ascent of patient centered technology and the digital and decentralized trials they’ve spawned has forever changed the landscape of clinical conduct.


The Definitive Guide to Digital Evidence Generation
According to Grandview Research, the hybrid and decentralized clinical trial (DCT) market will be worth more than 12 billion dollars by 2030. Sparked by the COVID-19 pandemic of 2020, the rapid ascent of patient-centered technology and the digital and decentralized trials they’ve spawned has forever changed the landscape of clinical conduct. Sponsors are increasingly turning to DCT platforms in alignment with the rise of Life Science and Software-as-a-Service (SaaS) solutions. For those who haven't made the jump yet, there are many questions, including "What is a decentralized clinical trial?" Find out with this in-depth guide to decentralized clinical trials. Uncover how they work, their benefits, and how they transform clinical development.


Use case: Implementing digital solutions to improve oncology trial experience & efficiency
A top 10 pharmaceutical company looked to Medable to streamline participant burden and enhance site and team experience in oncology trials, while establishing a scalable template for future trials in oncology and other areas.

Report: Medable named #1 Leader in DCT for second year by Everest Group
For the second consecutive year, Medable has been named Leader decentralized clinical trial products per the Everest Group’s PEAK Matrix® assessment, which evaluates DCT products from 24 companies based on vision, capability and market impact.


ePRO Case study: Better options and outcomes oncology trials.
Medable worked with a top 5 pharma company to increase the safety of patients as anti-cancer treatments may cause pulmonary toxicity, ranging from asymptomatic radiological changes to respiratory failure, and is considered a common side effect.
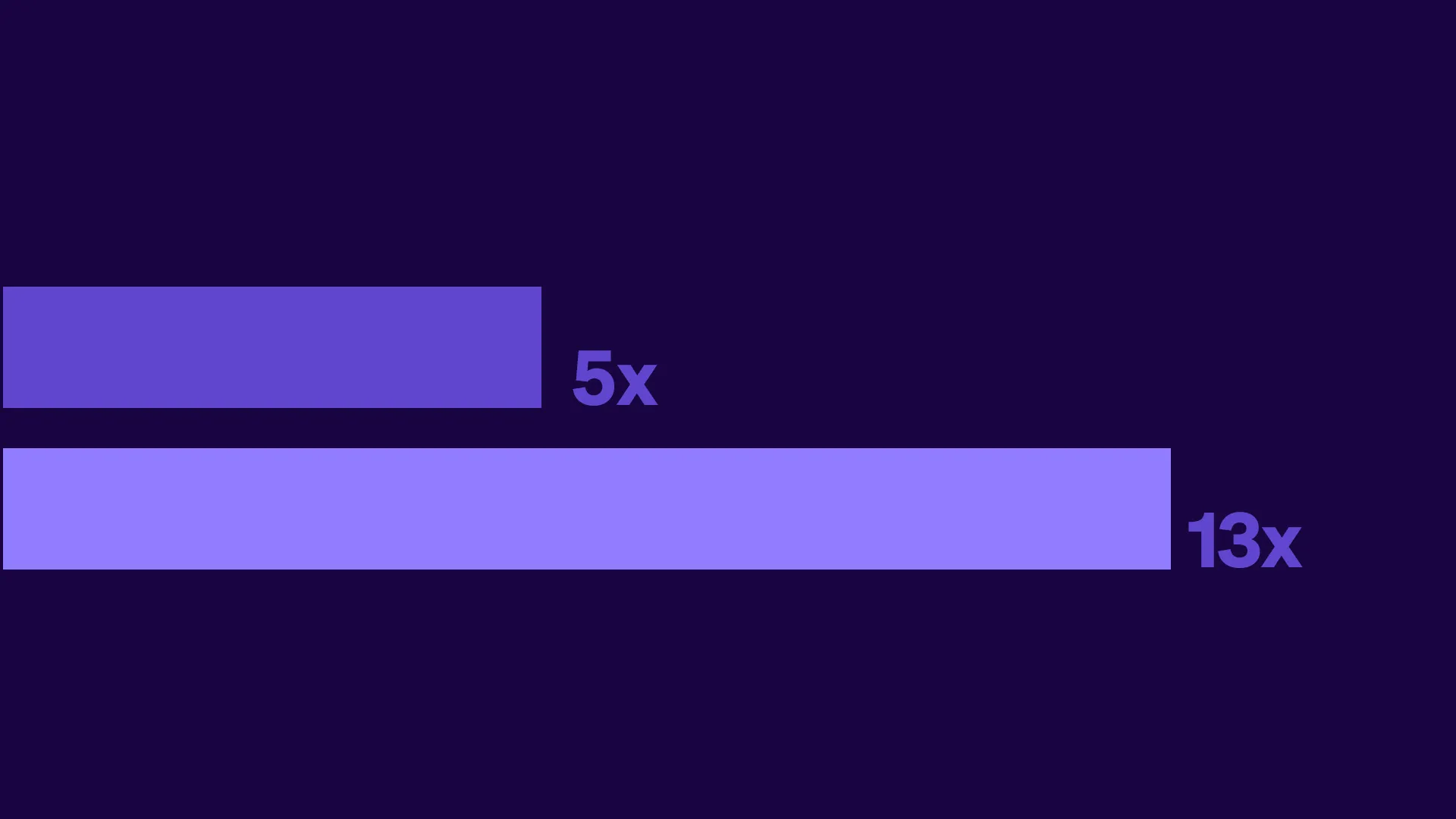

Tufts CSDD Impact report: Decentralized and hybrid trials deliver greater ROI than traditional trials
The Tufts Center for the Study of Drug Development completed an analysis of its financial modeling study of decentralized clinical trials using real data from Medable-enabled studies. These findings net financial benefits ranging from 5x for Phase II and 13x for Phase III trials, equating to roughly $10 million ROI and $39 million ROI, respectively.




