Meet Medable
We’d love to get to know you. Take our introductory questionaire to share more about your interests and receive a gift on us.
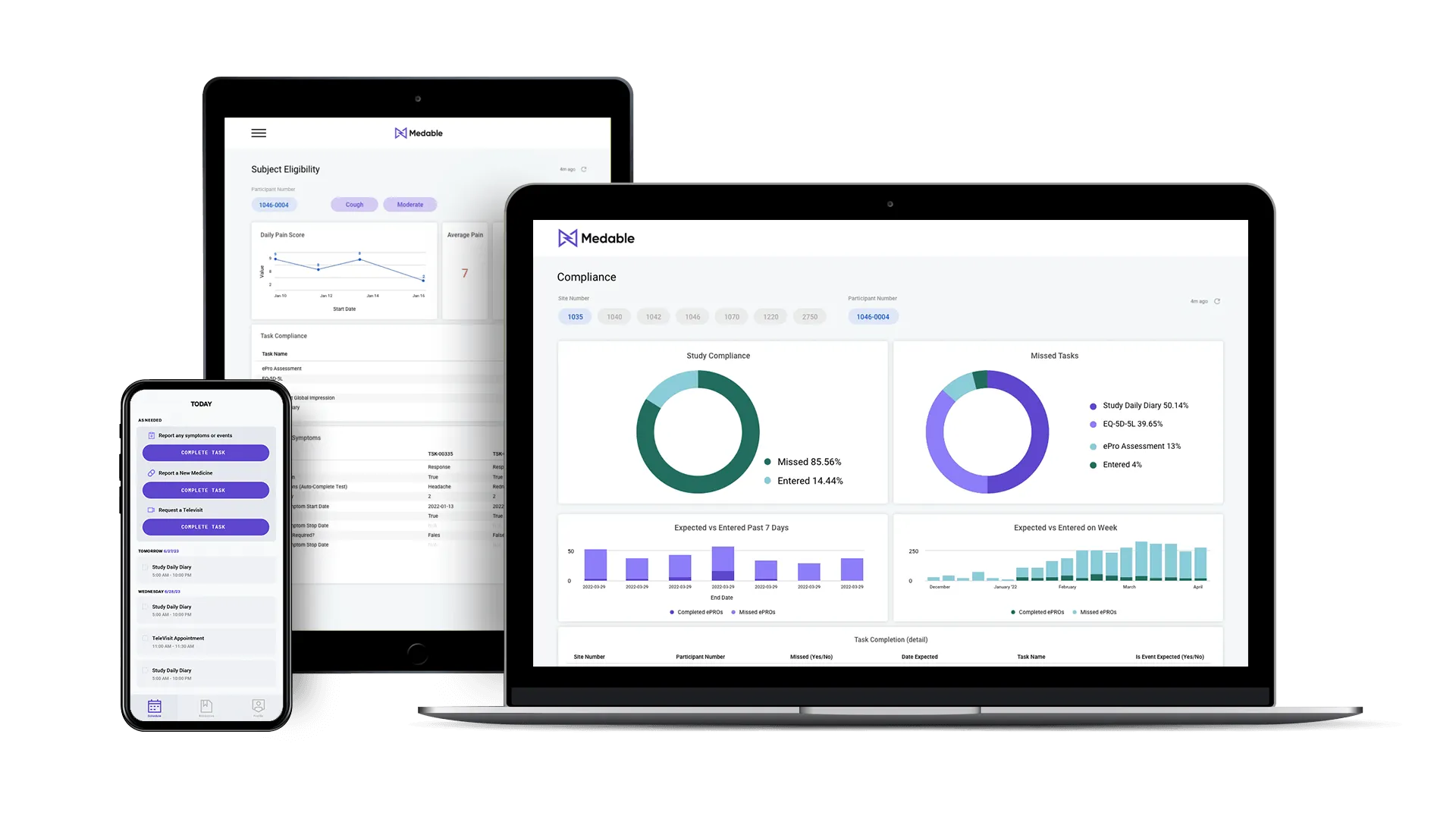
Join the top 14 of 20 Pharma companies who are streamlining trials, reaching patients around the world, and revolutionizing clinical research.
One platform, from start
to finish.
TeleVisit
Seamlessly connect patients and sites through scheduled or on-demand TeleVisits.
Total Consent
Our global eConsent solution offers wet ink and digital signature options so you can consent patients anywhere in the world.
eCOA+ and Connected Sensors
Our patient-centric eCOA+ collects precise digital measures to reduce variability, cut costs, and speed timelines while enhancing your trial findings.
Provisioned or Owned Devices
Devices that work for you and your patients. We offer a flexible solution, personal or provisioned, and web-enabled.
What clients are saying
Medable hired a third-party firm to get real feedback from customers across our trials. Here’s what they had to say about us.
Better compliance and faster study startup
A top-10 pharmaceutical company saw “increasing per-protocol data collection compliance” and “significantly reduced participant recruitment time.”
Increased oversight and scalability with eCOA and eConsent
A pharmaceutical group found success in “increased scalability, simplified clinical trial experience, and better oversight.”
Greater insights paired with an easy to use system
A top-10 pharmaceutical company achieved “streamlined user experience, aggregated data for greater insights, and seamless integration into participants lives.”
Accolades





Explore more of our featured content


Technology Overload: Addressing Site Challenges of Digital Trials
Katie madden, Digital & Process Optimization at GSK, joins Medable’s Andrew MacKinnon and Annie Hesslewood to discuss the importance of implementing a change management strategy coupled with a unified digital trial platform that streamlines eConsent, eCOA, sensors, and integration workflows into a single end-to-end experience.


Use case: Implementing digital solutions to improve oncology trial experience & efficiency
A top 10 pharmaceutical company looked to Medable to streamline participant burden and enhance site and team experience in oncology trials, while establishing a scalable template for future trials in oncology and other areas.


What it really takes to adopt eConsent across large pharma
Learn about the benefits of adopting consent management technology and best practices around developing change management and training programs to help sponsors, CROs and sites get the most out of eConsent.





